The era of RNA messenger: towards personalized medicine
Opportunities, challenges and prospects in the development of innovative mRNA therapies and vaccines


Media attention, countless laboratories around the world that focus on studies in this area, huge investments and scientific research at a global level that proceeds without slowing down. At the center is messenger RNA (mRNA), the main solution to combat the COVID-19 pandemic thanks to vaccines. But not only that, since the potential applications of mRNA are multiple and its use could transform the medicine of the future, allowing not only the treatment but also the prevention of pathologies that currently have no effective therapies.
What is messenger RNA (mRNA): meaning and origins
The messenger RNA molecule, acting as an intermediary, carries the instructions contained in DNA to direct the synthesis of specific proteins, without however modifying the original genetic information and, consequently, reduces the risks of long-term side effects.
mRNA-based therapies, which have become a widely debated topic by public opinion due to the pandemic, although innovative, have "roots" that date back to the last century. The first step dates back to 1961, when messenger RNA was discovered, simultaneously understanding the role played by the macromolecule which, as the carrier of a "message", allows all the cells of the organism to produce proteins. Subsequently, the world of research began to understand how to introduce mRNA into cells, however the first studies to use it - both for preventive and therapeutic purposes - were started 20 years later.

What are the opportunities offered by mRNA vaccines and therapies?
Continued and ongoing studies, with therapies that "look" at the causes of diseases and not at their symptoms, find application for a wide spectrum of pathologies - including those that are currently incurable - and can be personalized based on the specific information of the individual patients.
The increase in demand for mRNA therapies is due to the increase in chronic diseases, the aging of the population, the progress of scientific studies and sequencing and editing technologies, in addition to recent regulatory approvals such as that for the diffusion of mRNA vaccines “anti COVID-19” RNA.
The mRNA vaccines, characterized as demonstrated by the pandemic by speed of production and modification thanks to advanced technological platforms, can be administered using non-viral vectors such as lipid nanoparticles. In this way the risk of side effects is reduced, with this type of vaccine having proven to be as effective as and more than "traditional" protein-based ones in stimulating immunity.
The characteristics that make RNA a particularly useful molecule in medicine lie in its ability to carry information that makes human cells produce proteins, with an "information system" that is not capable of permanently modifying the genome or cells.
Even before the pandemic, mRNA-based therapies were being developed not only for viruses including Zika, but also for the treatment of different types of tumors such as, for example, breast cancer, lung cancer and melanoma, offering a valid and more effective alternative to traditional anticancer treatments - chemotherapy and radiotherapy first and foremost -, which take a long time to be administered and can have a significant negative impact on patients.
These therapies have potential that is extending towards rare genetic diseases – including cystic fibrosis and Duchenne muscular dystrophy – e autoimmune diseases, as well as preventative vaccines for infectious diseases such as HIV and hepatitis C.
The RNA therapeutics market is in a phase of rapid growth and involves various players including many innovative startups (as Biontech was in 2020 or, ten years or so earlier, Moderna). The global market including all mRNA therapies was valued at $5.2 billion in 2023, and is expected to be worth $17.2 billion by 2027 at a CAGR of 35%.
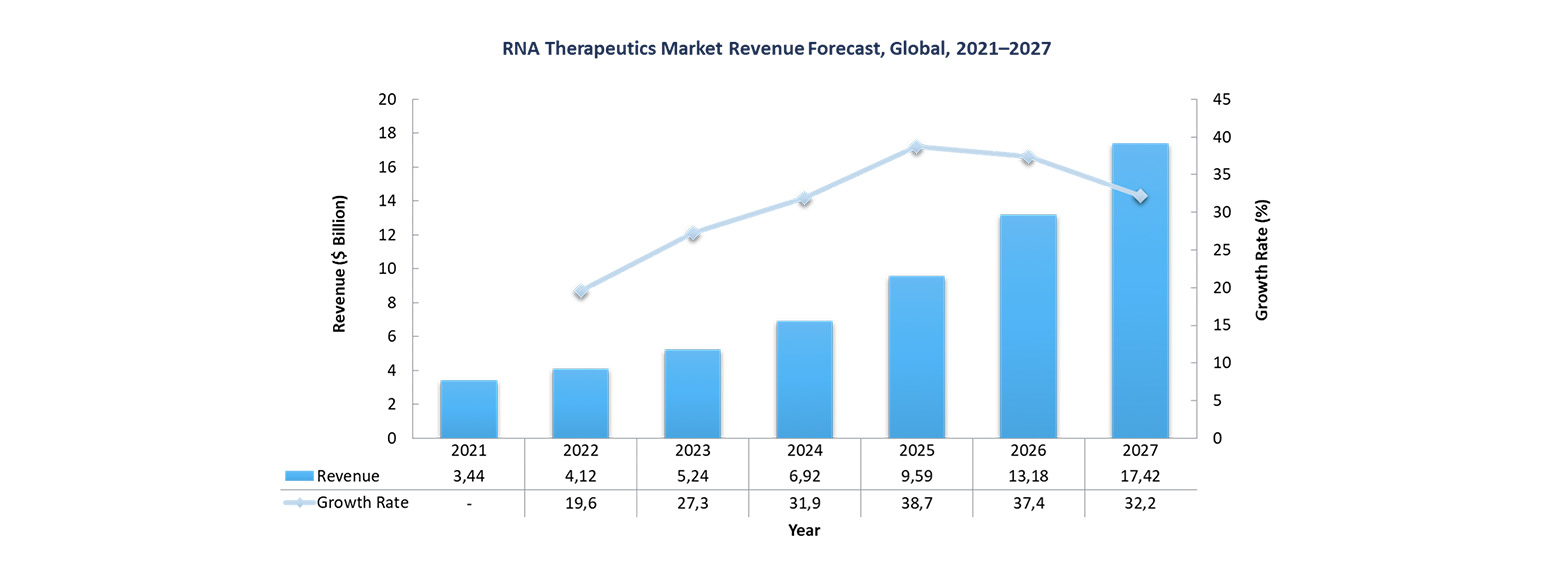
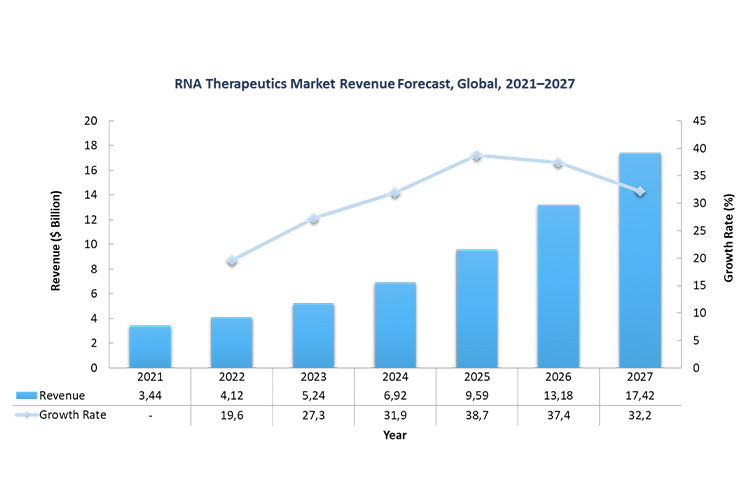
RNA-based therapies: challenges and prospects
The very nature of RNA, an intrinsically unstable molecule, requires the implementation of structured and standardized production processes, which have slowed down its development for applications in the healthcare sector. These efforts required in the production phase include the need to reduce contaminants to a minimum through a multi-stage purification system, correct management of the quantities of final product to be produced and equally correct distribution (transport and storage), which requires temperatures including between -80 and -20 degrees Celsius.
To address these problems and solve them, various actors involved - pharmaceutical companies, startups, research institutes, governments and international organizations such as WHO - are sharing best practices and adopting co-development approaches. A joint commitment that determines better and broader access to sequencing data and the related exchange between the parties involved.
For future developments it is expected that the digitalization of bioprocesses (the transformation of biotechnological products) will accelerate production to meet market raising demand. In fact, through process automation and data optimization with technologies such as artificial intelligence, digital twins and IoT sensors, it is possible to improve the traceability of materials by allowing processes to be simulated and monitored in real time.
